Understand the World
How to respond and avoid panic on supermarket shortages
Published
2 years agoon
By
lily
In 2020 and 2021, empty shelves were due to spikes in demand, as shoppers responded to lockdowns by buying more toilet paper, pasta and other consumables. This disrupted the usual rhythms of predictable supply chains. Apart from the first wave in March 2020, shortages were localised.
Now the shortages are due to supply-side problems and occurring (almost) nationally. As Omicron infections surge in every state apart from Western Australia, supply chains are being crippled by the sheer number of transport, distribution and shop workers now sick or required to isolate.



The major problem now is in transport and distribution. The Transport Workers’ Union says a third to half of Australia’s truck drivers are off work. Woolworths chief executive Brad Banducci said on Friday more than 20 per cent of distribution centre staff and more than 10 per cent of store workers are absent.
There are also problems in production, particularly in meat processing — an industry prone to the spread of COVID-19. Hundreds of workers in eastern states abattoirs are off work, according to Meat Industry Council chief executive Patrick Hutchinson. He has warned of severe shortages within weeks due to the lack of rapid antigen tests.
A self-fulfilling crisis
Then, of course, there is the response of shoppers to shortages (or the expectation of shortages). We’ve seen how this works multiple times: products disappear from shelves, people buy more in response. Fear of shortages becomes a self-fulfilling prophecy.
Coles has already imposed buying limits on certain meat items (except for WA) and warned customers to expect shortages for all of January.
Woolworths and ALDI have not (apart from limits on hard-to-get rapid antigen tests), but they might be forced to. That depends mostly on what happens in the next weeks in NSW, which plays a large role in national grocery logistics and where COVID-19 infections are surging.
What you can do
If you don’t have three weeks’ supply, and have both the space and money to stock, go for it. If not now, because there is a shortage, then in the next opportunity. This is not about panic-buying or hoarding. Not suggesting you buy a year’s worth of toilet paper or tinned food. Just always have enough so you can have peace of mind next time.


Supermarket restrictions
The pandemic has exposed the brittleness of just-in-time supply-chain management, which over decades honed the amount of stock held by manufacturers, wholesalers and retailers to a minimum. This was fine for maximising profits in good times. Now the times call for more of a just-in-case approach, with enough flexibility to avoid the system collapsing in a crisis.
Supermarkets have already made changes to avoid repeats of the supply crises of 2020 and 2021 by keeping more stock on hand. But this alone can’t solve the problem. The grocery business is competitive. Floor and refrigeration space is finite. They can’t afford to overstock.
What they can do is move to a more decentralised system for restricting quantities of items customers can buy when shortages do occur.
Every store can calculate safety stock levels to protect them from supply-chain fluctuations. When an item is about to go missing from the shelves, they shouldn’t have to wait for a decision from the central office to restrict quantities. They should be able to do it on the spot, while corporate headquarters work out alternatives.
When the problem is not lack of inventory but insufficient people to move products from warehouses to stores, the solution is visibility — letting costumers know about staff shortages, that there’s more than enough product on its way as soon as logistics allow, and that other stores are better supplied.


Cooperation is key
It is unlikely every store will be equally hit by labour shortages at the same time. Imagine evolving to a point where a Coles store with empty shelves informs shoppers the product is available two blocks away at the IGA.
For this to happen, of course, requires cooperation between competitors, and therefore easing of the usual anti-cartel rules that expressly prohibit collusion. But there is a clear precedent for this. In April 2020, the Australian Competition and Consumer Commission gave temporary authorisation to telcos, banks, medical suppliers and supermarkets to collaborate to ensure the supply of essential goods and services.
There is a clear case for supermarkets to cooperate now — and for the foreseeable future — with the Australian Retailers Association expecting the supply chain issues to continue for at least 12 months.

Both federal and state governments can help set the rules of engagement, and provide accurate and actionable information to give the correct dimension of the problem. They have, for example, data from past decisions such as the effect of Victoria’s restrictions on abattoirs in 2020.
If everyone is ready, doing what they can, we may reach a culture of resilience in Australia where empty shelves in supermarkets are but a bitter memory.
You may like
Understand the World
Hong Kong Speeds Up Article 23 Legislation in Full Swing, International Prospects Worrying
Published
1 month agoon
March 19, 2024By
lily
As China’s National People’s Congress (NPC) and Chinese People’s Political Consultative Conference (CPPCC) convene, the Hong Kong SAR government has accelerated the enactment of legislation on national security under Article 23 of the Basic Law, and the Legislative Council (LegCo) has convened additional plenary sessions. The Chief Executive, Mr Lee Ka-chiu, has reiterated the need to “complete the legislation as soon as possible”, which is undoubtedly aimed at further securing Hong Kong’s “second reunification”, and critics are worried that Hong Kong’s few remaining human rights and freedoms will be further eroded.

Accelerated Article 23 Legislation
Article 23 of the Basic Law of Hong Kong stipulates: “The Hong Kong Special Administrative Region shall enact laws on its own to prohibit any act of treason, secession, sedition, subversion against the Central People’s Government, or theft of state secrets; to prohibit foreign political organisations or bodies from conducting political activities in the Region; and to prohibit political organisations or bodies of the Region from establishing ties with foreign political organisations or bodies”. After Britain handed over sovereignty over Hong Kong to China in 1997, Beijing repeatedly expressed its hope that Hong Kong would enact legislation on its own in respect of Article 23 as soon as possible, but it was never successful in doing so.
In 2003, the Hong Kong government’s attempt to push for legislation on Article 23 triggered hundreds of thousands of people to take to the streets in protest, which was the largest rally since the handover of Hong Kong’s sovereignty. Due to public pressure, the legislation on Article 23 was shelved. After a gap of 21 years, a one-month public consultation on Article 23 legislation was held from 30 January to 28 February this year. According to Mr Lee, 98.6% of the respondents expressed support and positive views during the public consultation period, indicating that the legislation has a strong public mandate. Just over a week after the end of the public consultation period, the Hong Kong government submitted the “National Security Bill” to the Legislative Council for scrutiny.

It is worth mentioning that they were supposed to attend and participate in the National People’s Congress (NPC) and the Chinese People’s Political Consultative Conference (CPPCC) in Beijing, but they were suddenly summoned to return to Hong Kong on Tuesday night to attend a joint meeting of the LegCo committees on the legislation of Article 23 of the Basic Law on the following day. This reflects that these arrangements were made in a rush and were not planned in advance, most probably to create a sense of crisis for the legislation of Article 23 of the Basic Law as soon as possible. Generally speaking, a bill requires several rounds of debates in the Legislative Council and a special meeting for the first and second readings, and the whole process may take several weeks. However, the HKSAR government claims that as geopolitics is now more complex and volatile, and threats may come out of nowhere, it must plug the gap in national security and complete the legislative work as soon as possible.
A few days ago, Xia Baolong, Director of the Hong Kong and Macau Affairs Office (HKMAO), met with the Hong Kong National People’s Congress (NPC) and the Chinese People’s Political Consultative Conference (CPPCC) in Beijing, and they both expressed the hope that the Legislative Council (LegCo) would pass the legislation on Article 23 of the Basic Law as soon as possible, arguing that Hong Kong has waited for 26-and-a-half years since the handover, and claimed they believed that Hong Kong would have greater protection under the rule of law after the enactment of the legislation, which would enable its people to live in greater peace and solidarity. Some politicians said that as 15 April is the National National Security Education Day and the activities for the 75th anniversary of the National Day will be launched, it is estimated that Article 23 will have to be passed in time in April.

Is the national security law not enough?
Hong Kong’s previous governments have not succeeded in enacting Article 23, but this time, the legislation will be completed within the first term of Li Ka-chiu, which is a reference to the stormy changes in Hong Kong’s situation in recent years. Since Xi Jinping came to power in 2012, he set up the National Security Council and put forward for the first time the “Overall National Security Concept”, and the 20th National Congress even said that “national security is the foundation of national revival”. Against this background, the legislation of Article 23 of the Basic Law in Hong Kong is no longer simply a “constitutional responsibility” as it was described in the early days of the handover, but a top priority and a major political task for Zhongnanhai.
Under the principle of “one day early, one day gain” for local legislation on Article 23 of the Basic Law, the Hong Kong government gazetted the more than 200-page National Security Bill last week. The Legislative Council completed the First and Second Readings, and the Bills Committee commenced scrutiny of the Bill immediately. The Bill covers more than 20 national security charges and their offence elements derived from the newly enacted and amended existing legislation, as well as the penalties for various offences, extra-territorial legal effect, etc., with a number of new enforcement powers or reduction of legal procedures.
The Hong Kong National Security Law to be enacted in 2020 is targeted entirely at the “anti-China referral” campaign that will break out in Hong Kong in 2019 as a result of the amendment of the Fugitive Offenders Ordinance. The “four counts” set out in the law are secession, subversion of state power, organisation and implementation of terrorist activities, and intervention by foreign powers, but it is not a comprehensive national security law per se, with only the first two partially overlapping with, and not covering, Article 23. It does not cover the other five offences under Article 23, namely, treason, sedition, theft of state secrets, political activities of foreign political groups in Hong Kong and liaison between local political groups and foreign political organisations. The legislation on Article 23 this time around seeks to make up for the deficiencies in this respect.
Moreover, as the Hong Kong National Security Law has been much criticised in the international community, many countries and international organisations have demanded that the relevant law be repealed. If the Hong Kong government completes the Article 23 legislation this time, it is very likely that it will reduce the use of the “imperial sword” of the National Security Law in the future, so as to balance the previous criticisms. Comparing the charges of the “National Security Bill” with those of the current law, many of the penalties have increased significantly. Among them, the offence of seditious intent has increased from the current maximum imprisonment of 2 years to 7 years, and the offence of colluding with foreign forces can be sentenced to 10 years of imprisonment, which is undoubtedly a severe punishment, and it cannot but make people worry about the future of Hong Kong, which has already lost a lot of its democracy and freedom.
Gradual progress and accelerated delinking
The original plan for Chief Executive Li Ka-chiu to return to Hong Kong on Wednesday, March 6, has suddenly turned into an overnight rush to Hong Kong. Beijing’s wish for Hong Kong to speed up the completion of the Article 23 legislation has undoubtedly given a clear signal to the Western countries, led by the US, that the confrontation over “camping” has become more and more acute, and therefore the so-called national security loophole has to be plugged as soon as possible. In addition, the European Union has earlier passed a resolution that China and Taiwan are not subordinate to each other, and the United States has listed Hong Kong, China, Russia, North Korea, Iran and other countries as its overseas enemies. The speeding up of the legislation on Article 23 at this point in time undoubtedly reflects the gradual increase in the speed of China’s delinking from the West.
Outside China, criticisms and queries about the legislation on Article 23 have been expressed by civil organisations, academics or political figures. A few days ago, the former Speaker of the House of Representatives of the United States, Nancy Pelosi, claimed on a social media platform that the legislation on Article 23 has expanded the Chinese Communist Party’s attack on Hong Kong’s fundamental freedoms to an alarming extent. A member of the Hong Kong Legislative Council scrutinising the draft legislation on Article 23 of the Basic Law asked, for example, whether it would be an offence to “possess seditious publications” if a person kept an old Apple Daily newspaper at home as a souvenir. Officials said it would be an offence to possess seditious articles even after the law is passed, depending on whether the holder has a “reasonable excuse”. Once the law is passed, the Hong Kong authorities could criminalise the exercise of basic rights in the name of national security and suppress the voices of the pro-democracy camp.
Critics, including the US and UK governments, have said that the new Article 23 legislation will further narrow the freedoms of Hong Kong as a global financial centre, as many of the regulations and definitions are too broad and vague. The US State Department said in a statement in February this year that broad and vague definitions of “state secrets” and “outside interference” could be used to create fear of arrest and detention to stifle dissent. Currently, Western countries are closely watching the progress of the Article 23 legislation, which after all has the potential to affect the impact of foreign nationals, investments and company operations in Hong Kong. Once the Article 23 legislation is passed, more vaguely-defined provisions of national security laws with extraterritorial effect will further undermine the “one country, two systems” framework. There is no turning back from China’s disengagement with the West.
There is also public opinion that, given the tense situation between China and the Philippines, the Taiwan Strait, and especially the conflict between China and the Philippines in the South China Sea, and the heightened risk of war, Beijing wants Hong Kong to enact Article 23 as soon as possible, so as to prevent Hong Kong from becoming a “military loophole” in the South China Sea. Of course, there is no empirical evidence to show that there is any direct relationship between the legislation and the military conflict, except that the stability of Hong Kong will surely be favourable to the development of the Chinese Communist Party in various fields, and “protect” the Chinese Communist Party’s regime.
The Hong Kong National Security Law is already very powerful.
After the implementation of the Hong Kong National Security Law in 2020, it is obvious that Hong Kong is no longer the same as before. For more than three years, Hong Kong has been devoid of a media that criticises the government. The Apple Daily, which was seen as a promoter of pro-democracy protests, has ceased publication, and its top executives have all pleaded guilty and acted as prosecution witnesses in the trial of its president, Lai Chi-ying. The Internet media, which were accused of fuelling the rumour, are no longer in operation, and only a handful of Internet celebrities continue to discuss Hong Kong’s political situation overseas. Those who are still promoting democracy in Hong Kong, and those who are still discussing the government, are no longer as passionate as they used to be, but only provide some interpretations of things, and refuse to comment, and at the same time, their viewers have dropped significantly. Even personal opinions on social media platforms have been prosecuted as seditious, and Hong Kong people simply do not even discuss politics among friends.
It can be said that Hong Kong people prefer not to speak in the face of the situation.
Street demonstrations and protests against the government are no longer seen, and the Hong Kong government has claimed that it is now “moving from chaos to governance” and is beginning to “move from governance to prosperity”. In the Legislative Council, there are no dissenting voices against the government’s administration, and basically no motion that requires much discussion, amendment or rejection. Many civic groups and political parties that have existed for decades, such as the Hong Kong Professional Teachers’ Union, the Hong Kong Alliance in Support of Patriotic Democratic Movements of China, the Scholarism Movement, and the Civic Party, have been disbanded. It can also be said that those organisations that may hold opposing views to the government’s policies and that may be trying to disseminate or organise opposition voices in the community have been disbanded. It can be said that there is no more room for “endangering national security” in Hong Kong today.
So, what will be the effect of enacting legislation to implement Article 23 of the Basic Law?
Further Control of Thought and Speech
A careful analysis of the Hong Kong National Security Law and the Maintenance of National Security Bill reveals a fundamental difference. The former is a law enacted by China to be enforced in Hong Kong, covering the behaviour of anyone in the world, and has been challenged by Western societies for including anyone in the world. The latter divides its jurisdiction into three categories of people. Firstly, foreigners who have no direct connection with Hong Kong, and who are not mentioned in the Bill, apparently giving foreign governments no reason to criticise. The second category is residents of the SAR who are Chinese nationals, most of whom are living in Hong Kong, and they are naturally the target of the Bill. However, the third group are SAR residents who do not care where they live, and whose behaviour and speech outside Hong Kong are very often governed by the Bill. This is a matter of concern, especially for those born in Hong Kong who have emigrated to overseas countries.
All along, Hong Kong-born people have been given the right of abode to enter and leave Hong Kong freely, irrespective of where they have emigrated to or which nationality they have acquired. The identity card issued by the Hong Kong government is regarded as a document for travelling to and from Hong Kong, not a nationality declaration, and only a small number of people will voluntarily declare to the Hong Kong government that they have given up their right of abode in Hong Kong, but these people are regarded as permanent residents of Hong Kong with Chinese nationality. The number of such people in Australia is believed to be more than 100,000 people.
After the passage of the Bill, it is very likely that these people will be subject to the extraterritorial jurisdiction of the Bill, that is, if their words or behaviour expressed in Australia are regarded as having violated the Bill, they may be regarded as having violated the law and be subject to criminal liabilities when they return to Hong Kong. It can be said that for these Hong Kong immigrants, once they no longer return to Hong Kong or formally apply to Hong Kong to give up their right of abode, they will have to consider whether their remarks or behaviour in Australia have contravened the Bill.
Not only Hong Kong people in Australia, but also Hong Kong people in the diaspora all over the world are facing the same situation. Is this a kind of restriction on the speech and behaviour of these people? It can be said that these are some of the responses after the publication of the Bill. Some people think that the Australian government should confirm with the Hong Kong government whether this is the intention of the Bill, or to remind Hong Kong people that they should carefully consider giving up their Hong Kong permanent resident status.
If the Hong Kong government does not make this clear, or confirms that this is really the result, it is very likely that Hong Kong people overseas will be careful with their words, which is in fact a kind of threat to the freedom of speech. In any case, the passage of the Bill will probably trigger a new wave of emigration of Hong Kong people, or fewer foreigners will be willing to come to Hong Kong to work or travel, and it can be said that this may not necessarily bring positive consequences to Hong Kong.
Article/ translated from Issue 701, Editorial Department, Sameway Magazine
Photo/Internet

Following her global tour that began last year, Taylor Swift has become an economic force to be reckoned with, with each tour bringing not only musical enjoyment to her fans, but also new opportunities and challenges to local economies. Each of her tours not only brings fans the enjoyment of music, but also brings new opportunities and challenges to local economies.
Global Touring Helps Local Economies Soar
Taylor Swift’s The Eras Tour, which took place at Melbourne Cricket Ground (MCG) in Melbourne, Australia on February 16th, recorded a record attendance of 96,000 people. According to Forbes Australia, experts from RMIT University in Melbourne have estimated that the seven concerts held in Melbourne and Sydney, Australia by “Mildew” will record huge economic benefits. According to the estimation of Angel Zhong, Associate Professor of RMIT University’s Department of Finance, each fan will spend about AUD$900, which includes tickets, accommodation, transportation, merchandise and meals, and a total of 620,000 tickets were sold for the shows of “Mildew” in Australia, so it is estimated that the injection of at least more than half a billion dollars into the total economy of Australia.
In addition, Swift’s merchandise is not only sold at the concert venue, but also online or at local stores for fans around Australia. That’s a lot of money. In other words, it’s not just Victoria and NSW that benefit, but the rest of Australia as well. At the same time last year, the average occupancy rate for Melbourne hotels was nearly 70%, but almost 90% of hotel rooms were booked over the weekend of the Swift’s concerts, with visitors coming from both inside and outside Victoria, as well as from overseas, including from New Zealand. At the same time, February was the busiest February for low-cost carrier Jetstar, a strong boost to the surrounding economy given the cost of living crisis and the fact that February is usually a tough month for the airline industry.
In recent years, concert tours have become an important social phenomenon and economic driver. In the 20 U.S. cities Swift visited on her tour, economic growth was evident. In these cities, lodging inflation rose by 2.1 percentage points, and hotel occupancy and revenues increased dramatically. Chicago, for example, saw lodging prices rise by 3.1 percentage points due to Swift’s three shows, occupancy rates increased by 8.1 percentage points, and hotel revenue per available room rose by 59 percent.
According to a new analysis by Japanese investment bank Nomura Securities, Swift’s year-long global tour has truly reinvigorated economies everywhere. From hotels to retail, her visit has undoubtedly brought about a sizable increase in spending. But at the national level, this economic effect may be overstated, meaning that the boost to the overall macro economy may not be as huge as one might expect. But there’s no denying that Taylor Swift’s stunning “Generation Tour” sent waves around the world that not only drove her fans crazy, but also gave a significant boost to the economies of the countries she visited, and the impact continues to grow.

Bridging generations
One of the most talked about names in Australia this month is undoubtedly Taylor Swift. After months of waiting, young fans went wild at the concert, showing off the friendship bracelets they’d snapped up and chanting their fan slogans. And a closer look at those who took up (expensive) stadium seats in Melbourne and Sydney showed a lot of 40-, 50- and 60-somethings singing along – and they weren’t all with children. Statistically, while 45% of Swift’s American fans are millennials – like the 34-year-old herself – 21% are Gen Xers and 25% are baby boomers. That means nearly half of her millions of fans are probably over the age of 45.
Middle-aged Swifties aren’t shy about expressing their admiration. In the U.S., Attorney General Merrick Garland, 72, incorporates Swift’s lyrics into conversations and legal arguments, and reportedly has almost all of her albums on display in his office. 56-year-old Oscar winner Julia Roberts is also so enamored with her that the first concert she took her kids to was the 2015 Swift 1989 album, “The Best of Swift. Swift’s 1989 album tour. In Australia, AMP chief economist Sean Oliver, actor Toni Collette, former Foreign Minister Julie Bishop and academic Larissa Berendt have all declared themselves Swift fans.
Taylor Swift is undoubtedly the most successful pop star in the world today, and her cultural and economic influence is overwhelming: she has more number one albums on the Billboard charts than any other woman in history, and was the most played female artist on the music streaming platform Spotify last year, with concert videos that have yet to be officially released in the United States. The concert video was a surprise hit in the U.S. before it even officially aired – with record-breaking pre-sales of more than $100 million. Numerous articles have been written online attempting to analyze why and how Taylor has made such an impact. Experts from the fields of economics, linguistics, psychology, neuroscience, and music management have tried to explain this phenomenon.
Taylor’s success may be due to the fact that her music resonates with a wide range of people. Many of Taylor’s songs tell a story in just one song: mainstream pop fans love her accessibility and catchiness, teenagers feel listened to, music fans and critics recognize her talent, and even indie/punk rock fans love her. What sets her apart from her contemporaries is her ability to constantly reinvent herself, which has led to a longer career and a wider fan base.

The Rise of Female Power
In recent years, female fans have become an increasingly visible and powerful force in many areas of pop culture, media and entertainment, with last year’s Women’s World Cup in particular providing a whole new dimension to the portrayal of women. Taylor Swift has also been a longtime advocate of female empowerment and independence, and the themes of growth, love, and self-awareness in her songs have had a profound impact on all of us.
Last June, Kenyan football star Travis Kelce said he was a fan of Swift but did not know her. Later in September, the two officially dated, and Swift attended many of Travis Kelce’s games to show her support. The number of women attending football games in the US increased dramatically, and the number of women following football games on social media skyrocketed. This year, more female fans followed Taylor Swift to the 2024 Super Bowl, and many advertisers are targeting the female Gen Z and Millennial audience. It’s fair to say that Swift’s romance has indirectly changed the advertising strategy for traditional sports in ways that were not expected. It’s hard to believe that a commercial for women’s sanitary napkins would be featured on a football telecast, but it’s surprising.
With the popularity of feminism, today’s female celebrities need to ‘wake up’ to their true selves and become better people by transcending limitations. The documentary “Ms. Americana” shows this process in its entirety: Taylor’s previous life revolved around one standard – to be perceived as a “good person” – so she waved and smiled and dieted and dieted and carefully hid her political positions. And so she smiled and dieted and lost weight and carefully hid her politics. But after a series of disputes with Kanye West, Taylor decided to stop hiding herself and made the leap to her new self by speaking out against Trump, launching a sexual harassment lawsuit, and starting to support the rights of sexual minorities.
In Taylor Swift’s music, we can feel the rise of female power and the call for freedom. She is unafraid to express her feelings and opinions, whether it be about love, friendship, or social issues, she is unafraid to use her music to speak out. From her early pop music to her recent indie albums, ‘Folklore’ and ‘Evermore’, her music style has evolved, but she has always maintained her desire for real emotion. Her music has convinced us that women can not only have their own voices, but also write their own destinies in their own way. In her amazing 17-year career, Swift has now reached the pinnacle of her economic, cultural and political influence. This has seen her soar up the Forbes list of the world’s most influential women, from 79th in 2022 to 5th last year.
Swift and Billy Graham
Sixty-five years ago, the famous American evangelist Billy Graham visited Australia for 15 weeks in 1959, holding large-scale evangelistic meetings around the country. It is said that close to 145,000 people attended one night at the Melbourne Cricket Ground and many lives were changed. Australia has a population of about 9 million, and about 3 million Australians attended Billy Graham’s meetings. It can be said that Billy Graham’s evangelistic meetings had an impact on the whole of Australia. By this standard, Swift was certainly no match for Billy Graham.
However, from another perspective, what people today are looking for is no different from what Australians were looking for back then, they both want to find some enlightenment in their lives. Swift’s music and songs gave the audience a little satisfaction, and the audience showed extreme fervor, does it mean that today we can no longer find a convincing direction in life from leaders or visible role models? The search for meaning is not as important today, and while Billy Graham’s message may not reach as many people as it should, Swift’s music is still influential in many ways.
Japan and Singapore but not Hong Kong
It is worth mentioning that only Japan and Singapore were chosen as the two places to hold concerts for the Asian dates of the Times Tour, which made many Hong Kong fans feel disappointed. It is known that the reason for choosing to hold the concert in Singapore is that the Singapore Fund subsidizes and waives the venue rent, and the Singapore National Stadium can accommodate 55,000 spectators, which is in line with the requirements of the team.
At present, concerts around the world are very popular, and many celebrities are opening mega venues. Before the Kai Tak Sports Park was built, Hong Kong did not have any competition at the moment. However, it has been reported that Swift’s team had considered holding their concerts in Hong Kong’s 40,000-capacity stadium more than a year ago, but at that time, Hong Kong was in the midst of the “Dynamic Zeroing” of the new crown, and the Hong Kong government was not very enthusiastic in offering assistance, nor was it willing to waive or reduce the venue’s rental fee, which is far less favorable than that of the Singaporean government, and thus the people of Hong Kong were not able to have a chance to meet with Swift. Now the Hong Kong government has launched to organize the claimed grand activities to revitalize Hong Kong’s economy, but has never thought of organizing an international event like Swift tour, which also shows that the relevant officials in Hong Kong, lack of international vision, failed to see Swift is a global phenomenon.
When you think of the Lionel Messi controversy that happened in Hong Kong earlier this year, maybe it’s not the best idea for a big star to skip Hong Kong. Taylor’s rise to the pinnacle of power in pop music may itself be an example of how the female experience is gradually being illuminated: the secret of the teenage heart isn’t trivial or unimportant, it’s proof of having lived as a human being, and it can be an unstoppable whirlwind of emotions that can be a huge driver of the economy.
Author/Editorial Department
Photo/Internet

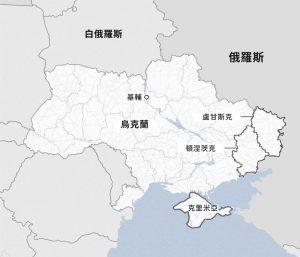
February 24th marks the second anniversary of the escalation of the crisis in Ukraine. The prolonged crisis has not only caused huge losses to the Russian and Ukrainian economies and people’s livelihoods, but has also had a serious negative impact on regional politics and security, the recovery of the world economy, global poverty reduction, food and energy security, and the ecological environment. On the 17th of February, the Ukrainian army, which is short of troops and ammunition, withdrew from the defence town of Avdeevka, which is regarded as the biggest change in the front line since May last year. At present, Russia and Ukraine are at a stalemate on the battlefield, and the tug-of-war between the two sides will continue.
The battle remains a stalemate
The crisis in Ukraine escalated on 24 February 2022, when Russia launched a special military operation against the country, and came at a time when the world was experiencing a three-century pandemic. The war on the front line has been virtually at a standstill for the past 14 months, with Moscow controlling nearly one-fifth of Ukraine’s territory, including the Crimean Peninsula, which it annexed in 2014. The war has caused hundreds of thousands of casualties, destroyed many cities, towns and villages, forced millions of people to leave their homes and left hundreds of thousands more living in occupied territories. Looking back at the two years since the outbreak of the crisis, the war has remained a stalemate, and the impact has continued to spill over, not only limited to the military confrontation between the two countries on the battlefield, but also extended to the game between countries and regions in the political, economic, cultural and other fields, which aggravated the evolution of the world’s pattern of the hundred years of changes, and further pushed the international strategic forces and pattern of in-depth adjustments.
At the time of the crisis, the international community generally believed that Ukraine would soon collapse, but it was met with Ukraine’s stubborn resistance, and the Biden administration of the United States immediately began to unite with its allies to provide Ukraine with a steady stream of arms and financial assistance. At the same time, unprecedented sanctions were imposed on Russia. Russia and Ukraine began to fall into a tug-of-war. In the middle of last year, the world was shocked by the Wagner incident in Russia. This made the world think that Russia was on the verge of collapse after more than a year of extremely difficult fighting, but unexpectedly, Ukraine’s counter-attack was extremely difficult, and to a certain extent, it also slowed down the assistance provided by the United States and its allies to Ukraine, and the two sides once again came to a deadlock. From October last year, when the Israeli-Palestinian conflict broke out again, the crisis in Ukraine changed again, and the Russian army started to take more ground attacks. On 17 February this year, the Russian army took full control of Avdeevka, which became another turning point.
Last Saturday, on the second remembrance of the Russian-Ukrainian War, Ursula von der Leyen, President of the European Commission, met with three other Western leaders – Italian Prime Minister Giorgia Meloni, Canadian Prime Minister Justin Trudeau, and Belgian Prime Minister Justin Trudeau – to mark the second remembrance of the Russian-Ukrainian War. Italian Prime Minister Giorgia Meloni, Canadian Prime Minister Justin Trudeau and Belgian Prime Minister Alexandre De Croix arrived in Kiev at the same time. In Kiev, they emphasised Europe’s firm support for Ukraine until its “ultimate freedom” is secured. As the Russo-Ukrainian war entered its third year, the Ukrainian forces were facing increasing challenges on the front line, not only in terms of troop strength, but also in terms of weaponry and even ammunition. Commitment and firm support from the Western world at this time is undoubtedly a “shot in the arm”. During their short visit to Kiev, Meloni and Trudeau will each sign a bilateral security agreement with Zelensky. However, US President Joe Biden’s plan to provide US$60 billion in new military aid to Ukraine, although passed by the Democratic Senate, is still being shelved by the Republican House of Representatives, casting a shadow over Ukraine’s hopes to defeat the Russian army, which is superior in numbers and equipment, in battle.
Echoes of World War II
Although the Russian invasion of Ukraine was two years in the making, and the human scale of the destruction was shocking to the world, the scale of the invasion cannot be compared to World War II. However, there are various similarities between the two wars, ranging from the style of street fighting and weapons to the history and background of the times. In particular, the root cause of the two wars was a dispute over “righteousness”, a clash of ideologies, which was manifested in the real world by the financial crisis that had a devastating impact on the whole world; there was a gap of about ten years between the crisis and the outbreak of the wars, as was the case in both 1929 and 2008. Major financial crises and wars are symptomatic of deeper structural problems in society – underlying structural movements that create these cracks on the surface.
The financial crisis did bring many changes to the world, with quantitative easing relief, zero interest rates and fiscal austerity by governments to minimise the damage caused by the crisis, but at a high cost, not least in terms of inflation and the widening of the general wealth gap, which laid the groundwork for populism, extremist ideologies and social unrest. In modern wars, the trigger of World War I was the Sarajevo Incident, while the trigger of World War II was, in a sense, the Treaty of Versailles, which was too oppressive and restrictive, and severely weakened Germany, and Hitler’s ideology that led to the outbreak of the war, and the blitzkrieg attack on Poland in 1939 when Germany tore up the United Nations’ agreement, heralding the prelude to the Second World War. In the view of contemporary political and historical scholars, the Ukrainian War was a war between a democratic regime and an authoritarian regime, a war between two opposing philosophies, which involved the upholding of the norms of international relations. Russia’s invasion of Ukraine is also seen by political economists as a proxy war between authoritarian capitalism and liberal capitalism.
Although Russia’s annexation of Crimea in 2014 is considered to be the starting point of the Russo-Ukrainian War, the large-scale military invasion launched two years ago was clearly the first large-scale invasion war on the European continent after World War II, and has dominated international public opinion and become the focus of international geopolitical tug-of-war ever since its outbreak. The Kremlin has confronted the West with accusations of wanting to put NATO on Russia’s doorstep, and has been seen as claiming to be imposing its way of doing things around the world. Two years ago, it launched a “special military operation” in the hope of a lightning victory over Ukraine, but it was clear that his Putin’s ambitions had been dashed. Today, however, the balance seems to have shifted in Putin’s favour. Russia has been unanimously condemned and sanctioned by the West, and President Vladimir Putin is wanted for war crimes in international courts, but criticism from the South is rarely heard, and in the aftermath of the war, closer ties with China, Iran, and North Korea have provided Putin with diplomatic leeway and assistance in the international arena, serving as a platform for counterbalancing the West. Russia is embracing the prospect of a long war that the government believes can be sustained, and is taking advantage of the Ukrainians’ quicker depletion and the fatigue of its allies. It is difficult to say whether such a situation will allow Russia to continue to grow larger, and it is impossible to completely rule out the possibility of a wider war as a result of the friction. As in the case of World War II a hundred years ago, this is unexpected but reasonable.
The latest response from European countries
As a lesson learnt from the Second World War, European countries dare not be careless. After all, once Ukraine is defeated, it is hard to say that Putin will not “open his mouth wide”, and his ambition to regain the glory of the Tsarist Russia era may be on the verge of emergence, and then European countries will have to protect themselves. It is for this reason that French President Jean-Marie Macron, after meeting with more than 20 European leaders on Monday, said in his latest speech that European leaders have agreed to set up a coalition to provide Ukraine with medium-range and long-range missiles and bombs. Macron said the key to European security is to defeat Russia in Ukraine, or at least not to lose, because Europe can not afford the price. The European Union has not ruled out sending Western ground troops to Ukraine, but there are still differences between the allies. Of course, Russia is also “not willing to show weakness”, and has repeatedly warned that any deployment of Western troops in Ukraine will trigger a direct conflict between Moscow and the NATO military alliance. It seems that the situation has been brought back to the “Gordian knot” that existed two years ago, before Russia started its military conflict – NATO is the “thorn” in Russia’s side, and it is not a good idea to hit or even fight against Ukraine. NATO is a thorn in Russia’s side, and to go after Ukraine, even to the extent of fighting it, is the key to establishing its authority in the world. The war between the two sides is bound to continue, and neither side, nor the neighbouring Western countries, dare to act rashly, because a small act will often trigger an unpredictable “butterfly effect”.
Difficulty in opening the final chapter of peace
It has been two years since the outbreak of the Ukrainian crisis, and the aversion to war and the search for a peaceful solution have been festering around the world. At present, there are only sporadic glimmers of peace, but no clear signs of easing of the situation. Russia and Ukraine have gradually adapted to the situation. The entire Russian state has gradually turned to a wartime system, various economic indicators have rebounded, the military industry is fully supplying the front line, and the supply of troops continues to be replenished, Russia’s GDP has risen instead of declining, which is even more surprising. Despite the economic sanctions imposed on Russia by the West, Russia’s trade with China, India, Brazil, North Korea and many other countries in the Middle East is developing rapidly, forming a new pattern of world economic co-operation. In Ukraine, even though the country is in full defence mode, the majority of the population does not accept negotiations and insists on continuing the war. Although the US and its Western allies have reduced their support for Ukraine due to internal political struggles and the Gaza conflict, they will not stop their support immediately due to ideological and geopolitical factors.
The latest meeting of the European Union shows that these countries are beginning to worry that if Ukraine is defeated, Russia will become a new force dominating Europe, endangering their own survival. The three Baltic states, Finland and Sweden, which have just joined the North Atlantic Treaty Organisation, are even more nervous about the Russian invasion. The former Soviet Union countries in Eastern Europe even believe that they will become the next target after Ukraine falls. The current situation of the Russian-Ukrainian war has made the people of these European countries support a larger-scale confrontation. Of course, Russia’s poor military performance in the past two years has also strengthened the determination and confidence of these countries to demand a war.
The Russia-Ukraine conflict is a watershed in the world’s development since the end of the Cold War and even the Second World War, and will trigger deep changes in Europe and the Eurasian region, as well as have a far-reaching impact on the future development of the world order. This war is not just a war between two countries, but the most important war in the 70 years of World War II.
Uncertainties
One of the great uncertainties facing the Ukrainian crisis right now is the 2024 U.S. presidential election. If Donald Trump wins the election in November and takes over the White House again, will he cut back on aid, or even change course and refuse to assist Ukraine? This huge uncertainty is a source of great concern for Ukraine and Europe. According to the information of the Kiel Institute for World Economics Research, as of January 2024, the European Union (EU) has provided Ukraine with nearly US$92 billion, while the United States has provided US$73 billion in various kinds of assistance, including arms and funds. It was only after numerous discussions and bargaining that the EU approved a US$54 billion aid program for Ukraine in February this year. However, a new US$60 billion aid program proposed by the Biden administration has not been approved due to bipartisan political differences in the US. Given the unique position of the U.S. military in the West, without the U.S., it would be difficult for Europe’s own military strength to help Ukraine counter Russia. In this sense, the outcome of the US election will likely be a key factor in determining the future course of the Ukrainian crisis.
At the moment, the chances of any meaningful talks between the two sides are slim to nonexistent. There is no sign that Putin and his followers have changed their initial goal of conquering Ukraine, and the Russian leadership seems bent on fighting to the bitter end. For both Russia and Ukraine, war remains the only option. Russia will be tenacious in its campaign of conquest. Ukraine will bravely defend itself. As long as either Russia or Ukraine fails to achieve a landslide victory in the coming months – an unlikely scenario – the war will continue. For some time to come, the crisis may be a long process of alternating hot and cold, slow and fast, with the other side shedding more blood and suffering more losses, maximising the drain on the other side’s resources, and forcing the other side to back down in a battle of wills and resilience. No one can say for sure what the outcome will be. The only thing we can say for sure is that no one on either side of the Russian-Ukrainian war is a winner.
Article/Editorial Sameway
Photo/Internet
What to explore ?

Australia’s Multicultural Future

Hong Kong Speeds Up Article 23 Legislation in Full Swing, International Prospects Worrying

Taylor’s Cyclone

Two Years of Ukraine Crisis

Aftermath of Messi’s absence from Hong Kong Friendlies
Fraudulent ivermectin studies open up new battleground

Cantonese Mango Sago

NEMBC Arabic COVID 19 News – 14 June 2022
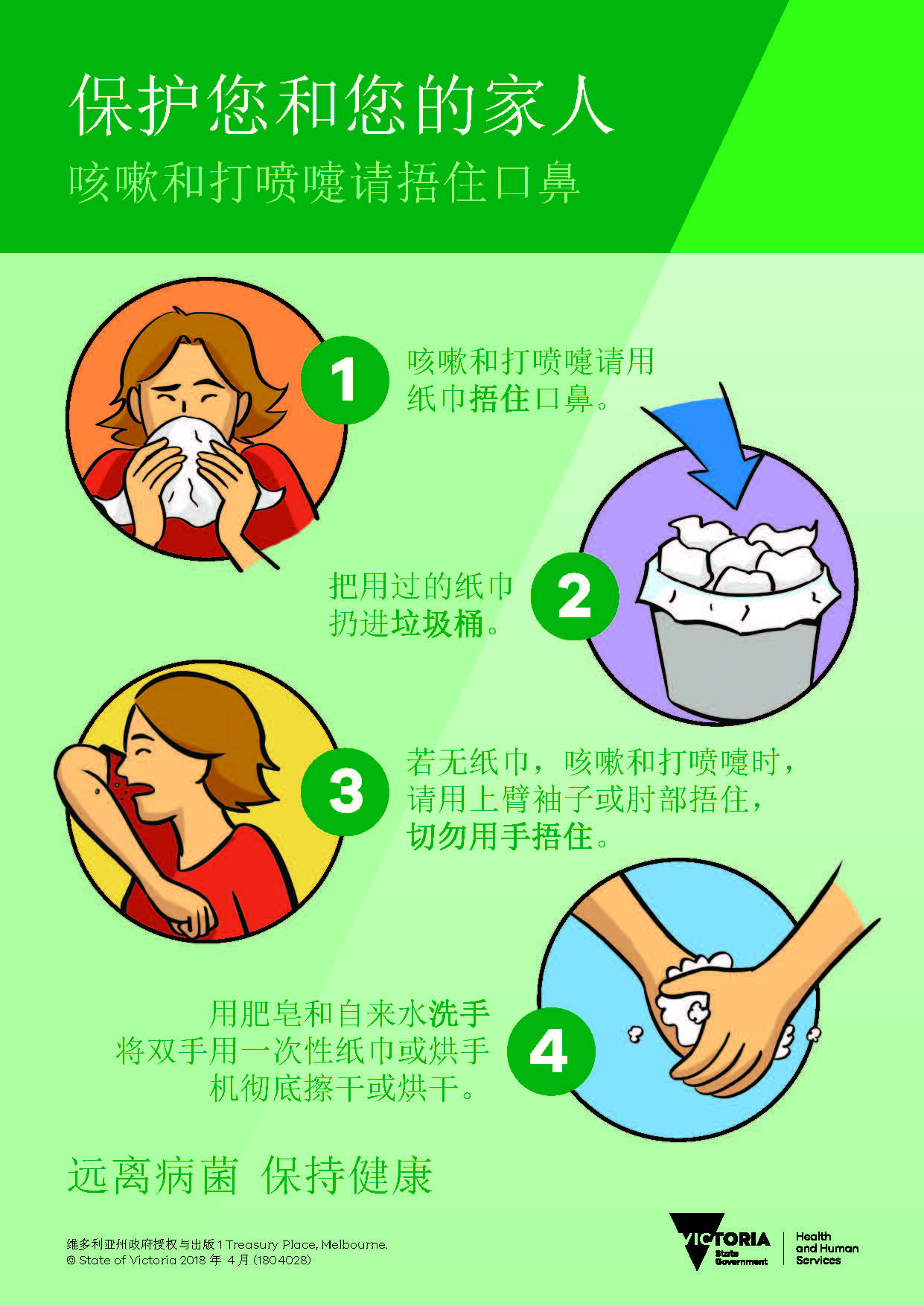
保护您自己和家人 – 咳嗽和打喷嚏时请捂住

FILIPINO: Kung nakakaranas ka ng mga sumusunod na sintomas, mangyaring subukan.
Trending
-
 COVID-19 Around the World3 years ago
COVID-19 Around the World3 years agoFraudulent ivermectin studies open up new battleground
-

 Cuisine Explorer4 years ago
Cuisine Explorer4 years agoCantonese Mango Sago
-

 Arabic2 years ago
Arabic2 years agoNEMBC Arabic COVID 19 News – 14 June 2022
-

 Cantonese - Traditional Chinese4 years ago
Cantonese - Traditional Chinese4 years ago保护您自己和家人 – 咳嗽和打喷嚏时请捂住
-

 Tagalog4 years ago
Tagalog4 years agoFILIPINO: Kung nakakaranas ka ng mga sumusunod na sintomas, mangyaring subukan.
-

 Uncategorized4 years ago
Uncategorized4 years ago如果您出現以下症狀,請接受檢測。
-
Uncategorized4 years ago
在最近的 COVID-19 應對行動中, 維多利亞州並非孤單
-

 Uncategorized4 years ago
Uncategorized4 years agoCOVID-19 檢驗快速 安全又簡單